BeiGene Enters Next Phase of Global Growth with Announcement of Second Quarter 2024 Financial Results and Corporate Updates
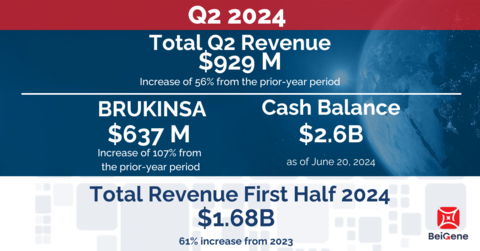
07.08.2024 - 13:06:00- Generated total revenues of $929 million, an increase of 56% from the prior-year period; reduced GAAP operating loss and achieved non-GAAP operating income
- Strengthened hematology leadership with global BRUKINSA revenues of $637 million, an increase of 107% from the prior-year period; advanced pivotal programs for BCL2 inhibitor sonrotoclax and BTK-targeted degrader BGB-16673
- Advanced innovative solid tumor pipeline of more than 15 investigational molecules, including ADCs, multispecific antibodies, and targeted therapies for lung, breast, and gastrointestinal cancers
- Strengthened global presence with opening of $800 million, 42-acre flagship U.S. biologics manufacturing facility and clinical R&D center in New Jersey and proposal to redomicile from Cayman Islands to Switzerland, an innovative biotech ecosystem for life sciences leaders and institutions
BeiGene, Ltd. (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global oncology company, today announced results from the second quarter 2024 and corporate updates that strengthen the Company for future global growth.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240807337131/en/
(Graphic: Business Wire)
“This was a tremendous second quarter and an inflection point as BeiGene achieved positive non-GAAP operating income with rapidly increasing global revenues and continued financial discipline. Having now reached this milestone, we will further build on our differentiated, strategic capabilities as a leading, global oncology innovator,” said John V. Oyler, Co-Founder, Chairman and CEO of BeiGene. “BRUKINSA is emerging as the BTKi class leader in the U.S. in new patient starts across all approved indications, demonstrating the strength of its clinical efficacy and safety data, and is the only BTKi to demonstrate superior efficacy versus ibrutinib in a head-to-head trial. With our leadership in hematology, we are working to expand into other highly prevalent cancer types, backed by one of the largest oncology research teams in the industry. With our continued growth in established biopharmaceutical hubs such as New Jersey and Switzerland, we are better positioned to reach even more patients with our innovative medicines.”
Financial Highlights
(Amounts in thousands of U.S. dollars)
|
|
|
Three Months Ended June 30, |
|
|
|
Six Months Ended June 30, |
|
|
||||||||||||||
|
(in thousands, except percentages) |
|
|
2024 |
|
|
|
2023 |
|
|
% Change |
|
|
2024 |
|
|
|
2023 |
|
|
% Change |
||
|
Net product revenues |
|
$ |
921,146 |
|
|
$ |
553,745 |
|
|
66 |
% |
|
$ |
1,668,064 |
|
|
$ |
964,036 |
|
|
73 |
% |
|
Net revenue from collaborations |
|
$ |
8,020 |
|
|
$ |
41,516 |
|
|
(81 |
)% |
|
$ |
12,754 |
|
|
$ |
79,026 |
|
|
(84 |
)% |
|
Total Revenue |
|
$ |
929,166 |
|
|
$ |
595,261 |
|
|
56 |
% |
|
$ |
1,680,818 |
|
|
$ |
1,043,062 |
|
|
61 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
GAAP loss from operations |
|
$ |
(107,161 |
) |
|
$ |
(318,715 |
) |
|
(66 |
)% |
|
$ |
(368,509 |
) |
|
$ |
(689,973 |
) |
|
(47 |
)% |
|
Adjusted income(loss) from operations* |
|
$ |
48,464 |
|
|
$ |
(193,051 |
) |
|
125 |
% |
|
$ |
(98,877 |
) |
|
$ |
(468,910 |
) |
|
(79 |
)% |
* For an explanation of our use of non-GAAP financial measures refer to the "Use of Non-GAAP Financial Measures" section later in this press release and for a reconciliation of each non-GAAP financial measure to the most comparable GAAP measures, see the table at the end of this press release.
Key Business Updates
(zanubrutinib)- U.S. sales of BRUKINSA totaled $479 million in the second quarter of 2024, representing growth of 114% over the prior-year period, with more than 60% of the quarter over quarter demand growth coming from expanded use in CLL as BRUKINSA continued to gain share in CLL new patient starts; BRUKINSA sales in Europe totaled $81 million in the second quarter of 2024, representing growth of 209%, driven by increased market share across all major markets, including Germany, Italy, Spain, France and the UK;
- Presented data from Arm D of the Phase 3 SEQUOIA trial evaluating BRUKINSA in combination with venetoclax in treatment-naïve (TN) patients with high-risk CLL and/or small lymphocytic lymphoma (SLL) with del(17p) and/or TP53 mutation as an oral presentation at the European Hematology Association (EHA) 2024 Hybrid Congress; preliminary data demonstrated an overall response rate of 100% in 65 response-evaluable patients and a rate of complete response (CR) plus CR with incomplete hematopoietic recovery (CRi) of 48%; and
- Presented new analyses highlighting improved progression free survival and response rates and a low usage of antihypertensive medicines for patients treated with BRUKINSA compared to other Bruton’s tyrosine kinase inhibitors (BTKis) used to treat CLL/SLL, including acalabrutinib and ibrutinib at the American Society of Clinical Oncology (ASCO) Annual Meeting and EHA.
TEVIMBRA® (tislelizumab)
- Sales of tislelizumab totaled $158 million in the second quarter of 2024, representing growth of 6% compared to the prior-year period;
- Presented new data from the Phase 3 RATIONALE-306 study evaluating TEVIMBRA plus chemotherapy in patients with advanced or metastatic esophageal squamous cell carcinoma (ESCC) at ASCO; and
- Received an update that the U.S. Food and Drug Administration (FDA) has deferred approval for tislelizumab in first-line unresectable, recurrent, locally advanced, or metastatic ESCC with a target PDUFA action date of July 2024 on account of a delay in scheduling clinical site inspections.
Key Pipeline Highlights
Hematology
Sonrotoclax (BCL2 inhibitor)
- More than 1,000 patients enrolled to date across the program;
- Completed enrollment in global Phase 2 trial in R/R mantle cell lymphoma (MCL) and continued enrollment in global Phase 2 trial in Waldenström’s macroglobulinemia (WM) and China-only Phase 2 trial in R/R CLL, all with registrational intent, as well as continued enrollment in global Phase 3 CELESTIAL trial in combination with BRUKINSA in TN CLL;
- At EHA 2024, presented data highlighting deep and durable responses with tolerable safety profile in Phase 1 studies in combination with BRUKINSA in R/R CLL/SLL and R/R MCL as well as results of additional Phase 1 trials demonstrating encouraging response rates, durable responses and manageable safety profiles as monotherapy in R/R WM, in combination with azacitidine in both TN and R/R acute myeloid leukemia, and in combination with dexamethasone in R/R multiple myeloma harboring translocation (11;14);
- Received FDA fast track designation for R/R WM; and
- Anticipating first subjects enrolled in Phase 3 programs in R/R CLL and R/R MCL in the fourth quarter of 2024 or first quarter of 2025.
BGB-16673 (BTK CDAC)
- More than 300 patients enrolled to date across the program; continued to enroll potentially registration enabling expansion cohorts in R/R MCL and R/R CLL; and
- At EHA 2024, presented data highlighting promising preliminary efficacy and safety in patients with R/R CLL/SLL; anticipating first subject enrolled in Phase 3 program in fourth quarter of 2024 or first quarter of 2025.
Solid Tumors
Lung Cancer
- Multiple randomized tislelizumab lung cancer combination cohorts with BGB-A445 (anti-OX40), LBL-007 (anti-LAG3) and BGB-15025 (HPK1 inhibitor) expected to read out in 2024;
- BGB-C354 (B7H3 ADC): Initiated dose escalation for the Company’s first internally developed ADC;
- BGB-R046 (IL-15 prodrug): Initiated dose escalation; this is a cytokine prodrug, leveraging protease-dependent release of active IL-15 in the tumor microenvironment and eliciting anti-tumor activity by promoting T and natural killer (NK) cell expansion; and
- Pan-KRAS, MTA-cooperative PRMT5 inhibitors and EGFR CDAC targeted protein degrader on track to enter the clinic in the second half of 2024.
Breast and Gynecologic Cancers
- BGB-43395 (CDK4 inhibitor): Continued dose escalation in monotherapy and in combination with fulvestrant and letrozole in the anticipated efficacious dose range with no dose limiting toxicities observed; more than 60 patients enrolled to date across the program; potential to share first readout of Phase 1 data in the fourth quarter of 2024; and
- BG-68501 (CDK2 inhibitor) and BG-C9074 (B7H4 ADC):Continued monotherapy dose escalation, with pharmacokinetics as expected and no dose limiting toxicities observed.
Gastrointestinal Cancers
- Tislelizumab combination cohorts with LBL-007 (anti-LAG3) in ESCC reading out in 2024;
- BLA accepted by the NMPA for zanidatamab for the treatment of second-line biliary tract cancer; and
- CEA ADC, FGFR2b ADC and GPC3x4-1BB bispecific antibody on track to enter the clinic in the second half of 2024.
Immunology & Inflammation
- Initiated clinical development of BGB-43035 (IRAK4 CDAC) with potential to induce deeper and faster IRAK4 degradation with stronger cytokine inhibition than competitors; this is the second targeted degrader from the Company’s proprietary CDAC platform.
Corporate Updates
- Opened flagship U.S. biologics manufacturing facility and clinical R&D center at the Princeton West Innovation Campus in Hopewell, N.J.; the facility includes 400,000 square feet of dedicated manufacturing space; and
- Announced intent to change jurisdiction of incorporation from the Cayman Islands to Basel, Switzerland, enabling the Company to deepen its roots in a global biopharmaceutical hub as it further executes on its global growth strategy to reach more patients around the world with its innovative medicines; this redomiciliation is subject to shareholder approval.
Second Quarter 2024 Financial Highlights
Revenue for the three months ended June 30, 2024, was $929 million, compared to $595 million in the same period of 2023, driven primarily by growth in BRUKINSA product sales in the U.S. and Europe of 114% and 209% respectively.
Product Revenue for the three months ended June 30, 2024, was $921 million, compared to $554 million in the same period of 2023, representing an increase of 66%. The increase in product revenue was primarily attributable to increased sales of BRUKINSA. For the three months ended June 30, 2024, the U.S. was the Company’s largest market, with product revenue of $479 million, compared to $224 million in the prior year period. In addition to BRUKINSA revenue growth, product revenues were positively impacted by sales of in-licensed products from Amgen in China and tislelizumab.
Gross Margin as a percentage of global product revenue for the second quarter of 2024 was 85%, compared to 83% in the prior-year period. The gross margin percentage increased primarily due to proportionally higher sales mix of global BRUKINSA compared to other products in the portfolio.
Operating Expenses
The following table summarizes operating expenses for the second quarter 2024 and 2023, respectively:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
GAAP |
|
|
|
Non-GAAP |
|
|
||||||||||
|
(in thousands, except percentages) |
|
Q2 2024 |
|
Q2 2023 |
|
% Change |
|
Q2 2024 |
|
Q2 2023 |
|
% |
||||||
|
Research and development |
|
$ |
454,466 |
|
$ |
422,764 |
|
7 |
% |
|
$ |
382,509 |
|
$ |
363,735 |
|
5 |
% |
|
Selling, general and administrative |
|
$ |
443,729 |
|
$ |
395,034 |
|
12 |
% |
|
$ |
363,922 |
|
$ |
331,607 |
|
10 |
% |
|
Amortization |
|
$ |
— |
|
$ |
188 |
|
(100 |
)% |
|
$ |
— |
|
$ |
— |
|
NM |
|
|
Total operating expenses |
|
$ |
898,195 |
|
$ |
817,986 |
|
10 |
% |
|
$ |
746,431 |
|
$ |
695,342 |
|
7 |
% |
The following table summarizes operating expenses for the first half 2024 and 2023, respectively:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
GAAP |
|
|
|
Non-GAAP |
|
|
||||||||||
|
(in thousands, except percentages) |
|
Q2 YTD 2024 |
|
Q2 YTD 2023 |
|
% Change |
|
Q2 YTD 2024 |
|
Q2 YTD 2023 |
|
% Change |
||||||
|
Research and development |
|
$ |
915,104 |
|
$ |
831,348 |
|
10 |
% |
|
$ |
787,949 |
|
$ |
725,431 |
|
9 |
% |
|
Selling, gener @ businesswireindia.com | ||||||||||||||||||





